To truly drive change and push the clinical research industry forward, we all need to come to the table. That’s why open-source contributions and solutions are a large part of our mission at Atorus. In support of these aims, Atorus has developed and contributed to multiple open-source analytics packages intended to broaden the reach of R statistical software users in the clinical field.
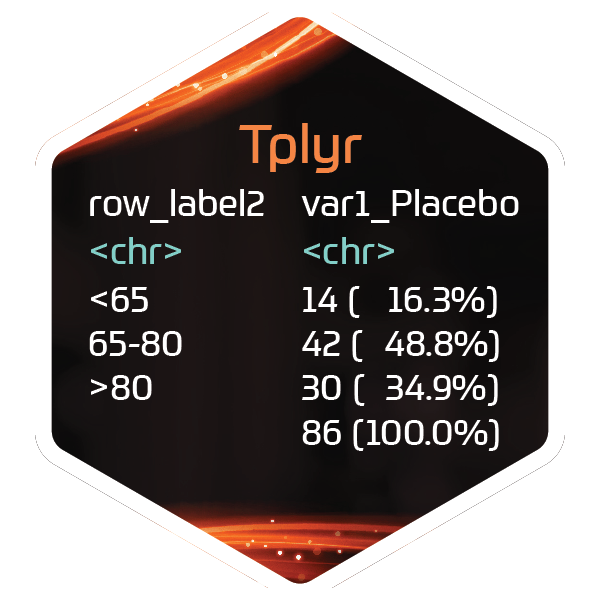
Tplyr
The Grammar of Clinical Data Summaries
Tplyr is a package dedicated to simplifying the data manipulation necessary to create static clinical reports for the pharmaceutical industry. A commitment to ease of use and flexibility guided every step of the development process for Tplyr, in addition to the vision of building a tool that did more than just summarize your data. Tplyr ultimately provides a framework for not just creating presentation-ready summary data, but also the traceability to allow you to drill down to much more.
Read more about Tplyr on GitHub and find it on CRAN.
Developed by Atorus Research.

pharmaRTF
An Enhanced RTF Wrapper for Use With Existing Table Packages
pharmaRTF allows users to leverage powerful R table packages to create industry standard format Rich Text Format (RTF) files. Using this platform, you can control document properties, repeat column headers across packages, include multilevel titles and footnotes, and more to create ICH-compliant formatted outputs.
Read more about pharmaRTF on GitHub and find it on CRAN.
Developed by Atorus Research.

xportr
Utilities to Output CDISC SDTM/ADaM XPT Files
xportr makes creating CDISC-compliant, submission-ready Version 5 transport files easy. Built on the underlying framework of the tidyverse haven package, xportr properly applies the necessary metadata to make your XPT files compliant with CDISC standards and ready for the Electronic Common Technical Document (eCTD). Built for clinical programming workflows, metadata can be pulled from the metacore package to make the XPT writing process seamless and simple.
Read more about xportr on GitHub and find it on CRAN.
Co-developed with GSK.

metacore
A Centralized Metadata Object Focused on Clinical Trial Data Programming Workflows
Utilizing metadata is essential to support efficiency and automation within clinical data workflows. Key to enabling this support is simplifying access to that metadata. metacore provides a single, in-memory interface for CDISC metadata that can be used by separate tools and packages within clinical programming workflows, so metadata can be read once and accessed as many times as needed thereafter.
Read more about metacore on GitHub and find it on CRAN.
Co-developed with GSK.
logrx (formerly timber)
A Logging Utility Focused on Clinical Trial Programming Workflows
Where do you get your log file when you’re programming in R? logrx offers you just the solution. logrx is built on the paradigm of letting SAS® be SAS® and letting R be R while making sure that we provide the right information to support an audit trail of program execution. With an organized collection of session information, packages accessed, and collection of messages, warnings, and errors, logrx plugs the gap of an R execution log and helps unify the SAS® and R programming processes.
Read more about logrx on GitHub and find it on CRAN.
Co-developed with GSK.

metatools
Enable the Use of “metacore” to Help Create and Check Data Sets
With packages like admiral to support ADaM programming workflows — and SDTM packages soon to follow — some programming is relevant to the life cycle of SDTM through tables, figures, and listings (TFL) as a whole. metatools is meant to be a home of metadata access functions that might be universally useful when working with CDISC data. Whether you’re doing compliance checks of data with your metadata, converting CT controlled variables to factors, or joining a SUPP back to a parent, metatools leverages metacore to automate these activities for you.
Read more about metatools on GitHub and find it on CRAN.
Co-developed with GSK and Roche.
pharmaverse
Atorus is just one part of the open-source analytics community supporting clinical programming efforts. Our packages and solutions are part of the larger pharmaverse, a curated, opinionated pharma stack of open-source R packages to enable clinical reporting from CRF to eSubmission, backed by a community of passionate individuals and organizations committed to co-creating efficiency in our mission to improve health.
Learn more at pharmarverse.org.